CMSE Presentation Series: Dr. Michael Buchmeiser
Professor Dr. Michael Buchmeiser gave 2 lectures during his upcoming visit to the University of Florida:
February 13, 2014 - Rhines 125 - 12:00pm
"Contributions of Olefin Metathesis to Material Science"
(MSE)
February 14, 2014 - Leigh 309 - 2:00pm
"CO2- and Metal-Protected N-Heterocyclic Carbenes as Truly Latent Catalysts in Polymerization (Organo-) Catalysis" (Chemistry)
See the announcement poster here.
Contributions of Olefin Metathesis to Material Science
Michael R. Buchmeiser, University of Stuttgart, Germany
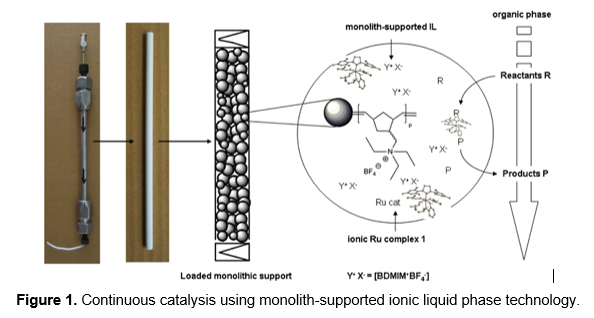
This presentation will address the impact of metathesis-based polymerization techniques such as ring-opening metathesis polymerization (ROMP), 1-alkyne polymerization and the cyclopolymerization of diynes on polymer-based material science. Along with aspects of polymerization and photo-catalysis, the design and tailor-made synthesis of functional polymeric materials will be addressed. Examples include stationary phases for selective metal extraction, analytical- and preparative-scale chromatography and large-volume applications such as the downstream processing of fermentation broths. Furthermore, functional supports for molecular, continuous heterogeneous catalysis (Figure 1) and continuous electro(bio-)catalysis will be presented. Finally, the synthesis of nanoparticle-reinforced polymeric, porous scaffolds from biodegradable monomers and their use in regenerative medicine will be demonstrated.
Krause, J. O., Zarka, M. T., Anders, U., Weberskirch, R., et al., Angew. Chem. Int. Ed. 2003, 43, 5965; Mayr, M., Wang, D., Kröll, R., Schuler, N.; M. R. Buchmeiser, Adv. Synth. Catal. 2005, 347, 484; Scheibitz, B.; Prager, A.; Buchmeiser, M. R. Macromolecules 2009, 42, 3493; Buchmeiser, M. R. Chem. Rev. 2009, 109, 303; Löber, A.; Verch, A.; Schlemmer, B.; Höfer, S.; Frerich, B.; Buchmeiser, M. R.; Angew. Chem. Int. Ed., 2008, 47, 9138; Wang, D., Wurst, K., Knolle, W., Decker, U. M. R. Buchmeiser, Angew. Chem. Int. Ed. 2008, 47, 3267; Mavila, S., Buchmeiser, M. R., Macromolecules 2010, 43, 9601; Bandari, R., Buchmeiser, M. R., Analyst 2012, 137, 3271; Autenrieth, B., Frey, W., Buchmeiser, M. R., Chem. Eur. J. 2012, 18, 14069; Autenrieth, B., Willig, F., Pursley, D., Naumann, S., Buchmeiser, M. R., ChemCatChem 2013, 5, 3033; Ferraz, C. P., Autenrieth, B., Frey, W., Buchmeiser, M. R., ChemCatChem 2013, doi: 10.1002/cctc.201300751; Sudheendran, M., Horecha, M., Kiriy, A., Gevorgyan, S. A., et al., Polym. Chem. 2013, 4, 1590; Unold, J., Wang, D., Frey, W., Buchmeiser, M. R., Polym. Chem. 2013, 4, 4219.
CO2- and Metal-Protected N-Heterocyclic Carbenes as Truly Latent Catalysts in Polymerization (Organo-) Catalysis
Michael R. Buchmeiser, University of Stuttgart, Germany
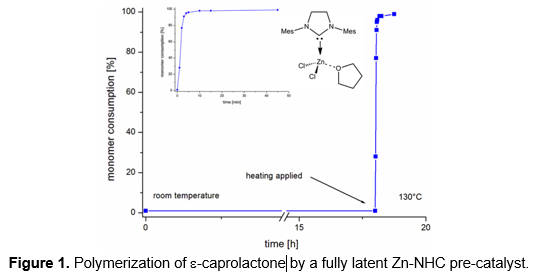
Far from being mere ligands for transition metals, N-heterocyclic carbenes (NHCs) are ever more recognized as powerful organocatalysts on their own and can act both as nucleophiles and bases. The flexibility with regard to ring-size, N-substituents and backbone, as well as the relative ease of preparation have won NHCs a prominent position in polymerization chemistry. However, disadvantages of free NHCs are the limited stability and storability and also the awkward handling of free carbenes since they are often of oily consistence. Consequently, much effort has been put into compounds that exist in the form of NHC-adducts or complexes but can release the active NHC after warming (“protected NHCs”) or by solvent effects. Here we describe differently substituted CO2- and metal-salt protected NHCs and their use as fully latent pre-catalysts in the polymerization of methyl methacrylate, various lactames, e-caprolactone, and for the synthesis of poly(urethane)s. Mechanistic aspects will be discussed. Also, the realization of room temperature-stable, latent resin systems based on one-component systems for reaction transfer molding applications, e.g., for the synthesis of fiber-matrix composites, will be addressed. Finally, cationic Ru-NHC complexes that serve as truly latent, UV-triggerable pre-catalysts for ring-opening metathesis polymerization will be presented.
Bantu B., Pawar G. M., Decker U., Wurst K., Schmidt A. M.; Buchmeiser M. R.: Chem. Eur. J. 15, 3103 (2009); Bantu B., Schmidt A. M.; Buchmeiser M. R.: Eur. J. Inorg. Chem., 1970 (2009); Naumann S., Epple S. E., Bonten C.; Buchmeiser M. R.: ACS Macro Lett. 2, 609 (2013); Naumann S., Schmidt F.-G., Schowner R., Frey W.; Buchmeiser M. R.: Polym. Chem. 4, 2731 (2013); Naumann S., Schmidt F. G., Frey W.; Buchmeiser M. R.: Polym. Chem. 4, 4172 (2013); Naumann S., Schmidt F. G., Speiser M., Böhl M., Epple S., Bonten C.; Buchmeiser M. R.: Macromolecules 46, 8426 (2013); Wang D., Wurst K. and Buchmeiser M. R.: Chem. Eur. J. 16, 12928 (2010); Wang D., Wurst K., Knolle W., Decker U., Prager L., Naumov S.; Buchmeiser M. R.: Angew. Chem. Int. Ed. 47, 3267 (2008).
|